About Our Work
Natural products are the major source of drugs, spanning antibiotics, painkillers, and cancer therapies. Nature has a fascinating array of enzymes that create this incredible diversity at room temperature using water as the solvent. From a chemist’s point of view, biosynthetic enzymes are really just protein-based catalysts that have been exquisitely evolved to accomplish these amazing feats.
The Lowell group seeks to not only identify medicinally important new natural products, but also understand the biosynthetic pathways responsible for their production. The enzymes identified in these pathways can in turn be used as catalysts in chemical synthesis. We harness these biocatalysts to synthesize and derivatize complex natural products to create new and improved medicines.
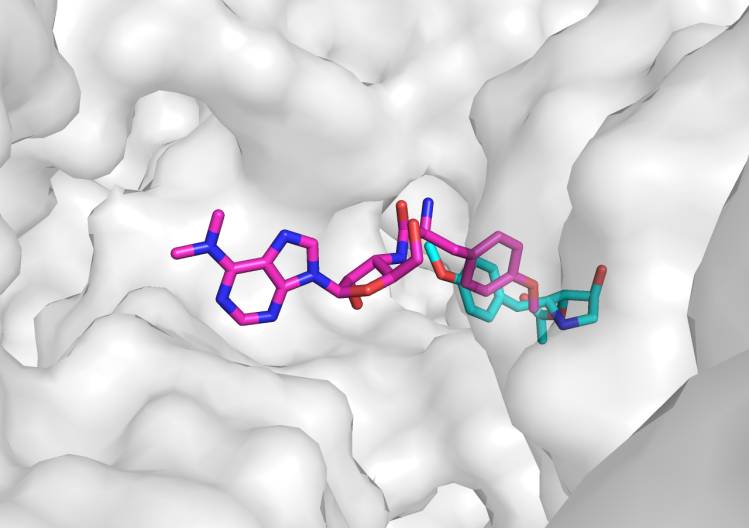
To meet the growing challenge of antibiotic resistance, we propose bidentate antibiotics, which are antimicrobials where two distinct chemical entities are covalently but flexibly linked in order to best engage their respective target sites simultaneously. We hypothesize that this two-pronged inhibition strategy will enhance antibiotic efficacy through stronger binding and synergistic interactions between two established and targetable interfaces, leading to more effective, broad-spectrum treatments against resistant pathogens such as MRSA and A. baumanii. By prioritizing binding proximity, feature analysis for linker attachment point, and complementary action, we believe the full potential of a hybrid antibiotic strategy – a truly bidentate antibiotic – will be realized.